1.Breating difficulty
2.skin sores, which may become a skin ulcer that heals very slowly
3.stuffy nose, runny nose, and nosebledds.
4.swallowing difficulty
TEST OVERVIEW for the Coronavirus(COVID-19)test
PERFORMANCE CHARACTERISTICS
The COVID-19 lgG/lgM Rapid Test (Whole Blood/Serum/Plasma) has been evaluated with the 126 samples obtained from patients exhibiting pneumonia or respiratory symptoms. The results were compared to Fluorescent Real Time Polymerase Chain Reaction (RT-PCR) or clinical diagnosis (including chest Computed Tomography and clinical signs etc.) of [Diagnosis and treatment of novel coronavirus pneumonia."
IgG/IgM Rapid Test results compared to SARS-CoV-2 RT-PCR results:
The sensitivity of lgM only test is 89.2% (100/112) and specificity is 100% (14/14).
RT-PCR Method testing was included as clinical site method. Nucleic acid was detected by probe fluorescence PCR. This product contained primers and probes (ORF1ab gene and N gene strains of coronavirus) and internal controls (housekeeping gene beta Globin gene sequences) used in RT-PCR for the in vitro qualitative detection of SARS-CoV-2 RNA in nasopharyngeal swab specimens. The novel coronavirus2019-ncov specific probe was respectively labeled with FAM fluorescence and ROX fluorescence, and the internal standard gene was labeled with VIC fluorescence. The minimum detection limit of the fluorescent RT-PCR assay is 10 copies/reaction.
LIMITATIONS
Coronavirus(COVID-19)test Rapid Test,Coronavirus Test Kit,Coronavirus Test,Covid-19 Test Dongguan Smart Furniture Co.,Ltd , https://www.smtfurniture.com
The IHS Technology Photovoltaic Micro Inverter and Power Optimizer Report predicts that revenue from smart communication components will rise from $100 million in 2015 to nearly $500 million in 2020.
Corres Gilligan, IHS Technology Research Manager, said: "Suppliers are shifting their business model from selling independent products to selling off-the-shelf integrated models. It will help capture emerging markets, increase sales channel efficiency and reduce customer costs."
The IHS technical report predicts that micro-inverters and power optimizers will increasingly be installed in factories in the form of AC and smart components.
Global Trends and Drivers The United States is the largest market for micro-inverters and power optimizers, but Asian and European markets such as China, Japan, Australia, Germany, France and the Netherlands are expected to face strong demand in the next five years.
IHS Technology predicts that in 2015, the smart communication component market will be less than 200MW, and by 2020 it will grow by more than 4GW, and integrated products will be favored in the global market.
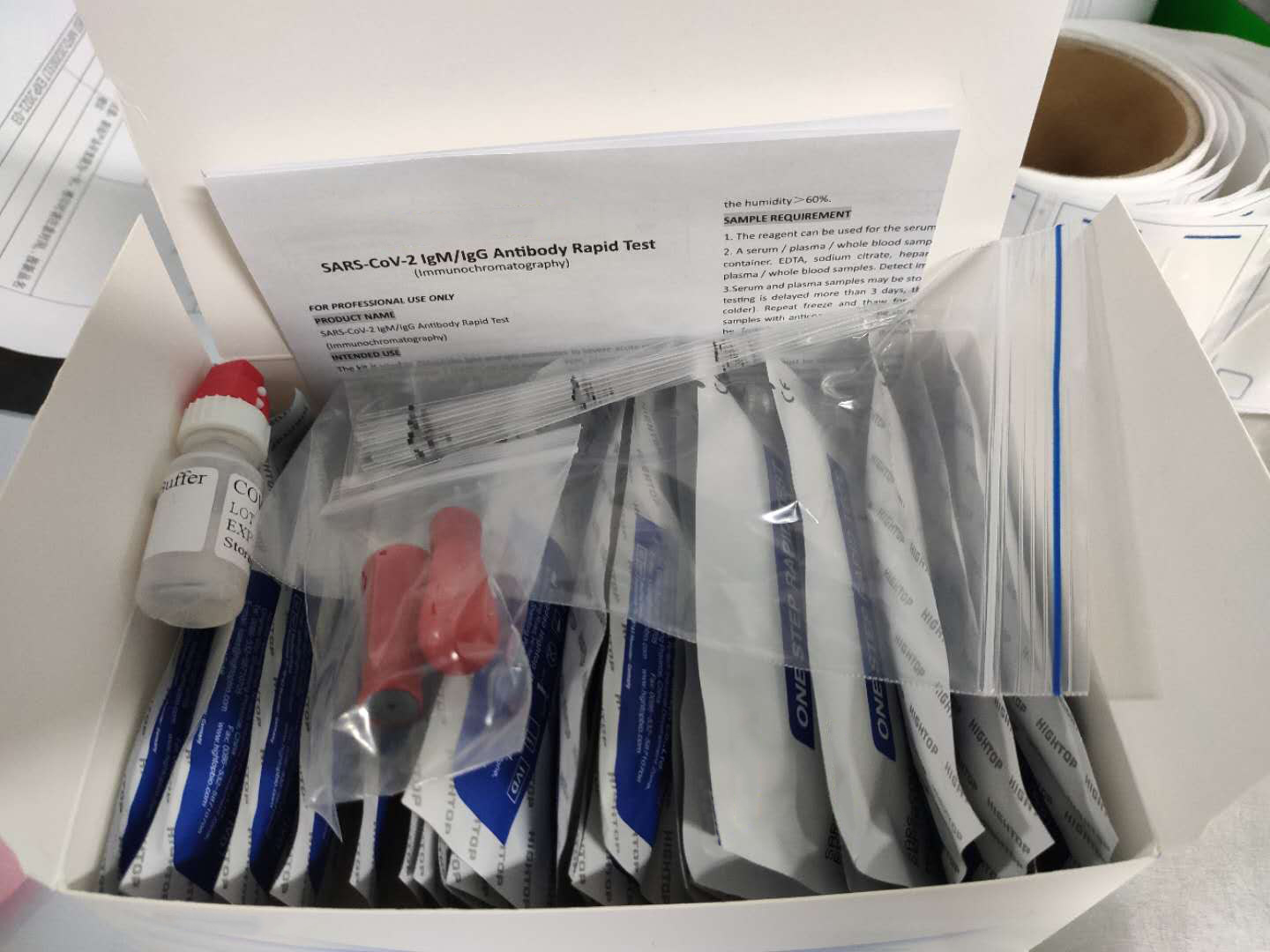
Description about Coronavirus(COVID-19)test Test
The Coronavirus(COVID-19)test is a solid phase immunochromatographic assay used in the rapid, qualitative and differential detection of IgG and IgM antibodies to the 2019 novel coronavirus in human whole blood, serum or plasma. The Coronavirus(COVID-19)test is intended for professionals within highly complex settings and or pharmacies. The test delivers rapid results between 2 and 10 minutes from individuals having signs and symptoms of SARS-CoV-2 infection
What conditions to do the Coronavirus(COVID-19)test?
COVID-19 RT-PCR Assay
Coronavirus(COVID-19)test
Positive
Negative
Total
Positive
103
9
112
Negative
0
14
14
Total
103
23
126
95% CI
Sensitivity
103/112
91.9%
(85.3% – 96.3%)
Specificity
14/14
100%
(76.8% – 100%)
Overall
117/126
92.8%
(86.9% – 96.7%)
The sensitivity of lgG & IgM test is 91.9% (103/112) and specificity is 100% (14/14).
The smart PV module market is expected to exceed 4GW in 2020
Abstract According to the latest analysis released by IHSMarkit in the UK, the business model of solar photovoltaic micro-inverters and power optimizer suppliers will be transformed from independent units to integrated systems, such as intelligent communication modules. IHS Technology Photovoltaic Micro Inverter and Power Optimizer...
According to the latest analysis released by IHS Markit in the UK, the business model of solar PV micro-inverters and power optimizer suppliers will be transformed from a stand-alone unit to an integrated system, such as an intelligent communication module.